Toxins From the Gut
Joseph Pizzorno, ND, Editor in Chief
When I was a third-year naturopathic medical student in 1973, one of my professors (Bob Carroll, DC, ND) started the first class of the year with the provocative statement, “Death begins in the colon!” I was skeptical. Having just finished basic sciences, the textbooks were very clear that the gut was a perfect protective membrane only allowing in nutrients the body needed. He then went on to lecture about how patients with digestive problems were more “toxic” and that by restoring proper digestive function and healthy flora, many experienced improved health. While I could see during clinic rotations that improving digestion and recommending eating natural yogurt helped patients with diverse diseases, I remained skeptical until I graduated in 1975.
During the first year of practice, I subscribed to several journals to read during those slow times building patient flow. I was quite diverse in my reading, ranging from the Lancet (which, though conventional medicine, had a surprising number of articles on nutrition and environmental medicine), the American Journal of Clinical Nutrition (back then, very conservative and dismissive of nutritional supplements), and a half dozen others. To my great surprise, I read a startling and controversial study reporting that in healthy animals, up to 1% of ingested proteins are absorbed intact and absorption increases to as much as 10% during severe gastrointestinal infection. My immediate thought was that “old” doctor Carroll might be right!
I have since followed with considerable interest our growing understanding of the role of the gut in health and disease. Integrative medicine clinicians are now well aware of how maldigestion, malabsorption, leaky gut, small bowel overgrowth of bacteria, inappropriate bacteria in the gut, and others contribute to and may even cause disease. When Michael Friedman, ND, asked me what topic I would like to present at the October 2014 Restorative Medicine in Santa Fe, New Mexico, I suggested I could dive into the research to see if this old idea about toxins from the gut was clinically relevant. Happily, he enthusiastically agreed. Following is what I found—and I think only the tip of the iceberg.
Gut Dysfunction
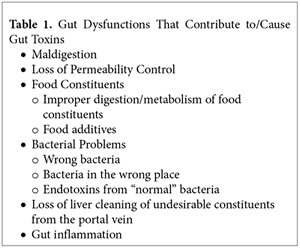 When thinking about toxins from the gut, many gut dysfunctions, as shown in Table 1, would appear to contribute to the problem. Maldigestion, loss of liver detoxification function, and gut inflammation are all important topics, and for space considerations, they are topics for future editorials.
When thinking about toxins from the gut, many gut dysfunctions, as shown in Table 1, would appear to contribute to the problem. Maldigestion, loss of liver detoxification function, and gut inflammation are all important topics, and for space considerations, they are topics for future editorials.
Basically, what appears to be happening is that most patients have the wrong bacteria in their gut and/or gut permeability control has been lost. This results in unhealthy metabolites (“toxins”) from gut bacteria entering into circulation. In fact, research has shown that up to one-third of the small molecules in the blood come from bacteria in the gut. Worse, however, is when a patient has overgrowth of particularly unhealthy bacteria, especially Gram-negative, the absorbed lipo-polysaccharides (LPS) are highly toxic with blood levels correlating with many chronic diseases.
Aggravating these problems are many food constituents that, when improperly digested and absorbed and/or not detoxified by the liver, cause diverse metabolic abnormalities—diamines in migraine being a typical example. Unfortunately, space limitations require moving this fascinating topic to another editorial as well.
Endotoxins
According to Wikipedia, endotoxin is defined as “any toxin secreted by a microorganism and released into the surrounding environment only when it dies.” Technically in the research literature, only bacterial LPS are considered “endotoxins.” LPS are the most studied and considered prototypic activators of innate immunity by gut bacterial products. These LPS represent 80% of the cell-wall mass of Gram-negative gut bacteria.
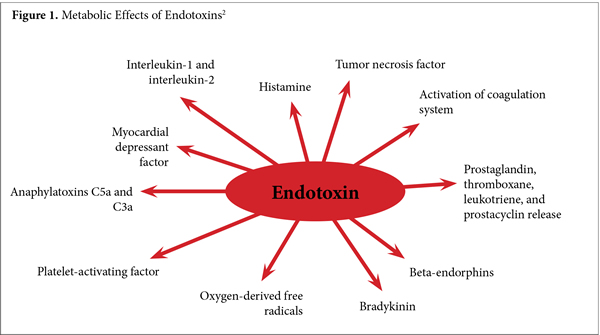
Here we use the more clinically relevant broader definition of endotoxin as “any metabolite or cell wall constituent released by gut bacteria that damages human physiology,” because a surprising 25% to 33% of the small molecules in human blood can be derived from gut bacteria.1 As can be seen in Figure 1, the effects of LPS and the many other endotoxins from gut bacteria cause substantive and diverse physiological dysfunctions.
 When endotoxins reach a high enough level in the blood, a threshold is reached called metabolic endotoxemia. Once this threshold is reached, several strong, dose-dependent disease associations become apparent, a few of which are shown in Table 2.
When endotoxins reach a high enough level in the blood, a threshold is reached called metabolic endotoxemia. Once this threshold is reached, several strong, dose-dependent disease associations become apparent, a few of which are shown in Table 2.
Interestingly, high levels of endotoxins also cause epigenetic changes similar to those seen in obesity, suggesting another mechanism for the known association between various gut flora and risk for obesity.7
There are many reasons for the increased levels of endotoxins seen in modern civilizations. Obvious causes are the overuse of antibiotics, increased incidence of Cesarean births, and lack of breast-feeding. Less obvious, perhaps, is stomach acid secretion suppression by proton-pump inhibitors and H2 blockers. The research is very clear that the use of these agents results in increased colonization of the gut by Clostridium difficile, thus substantially increasing the release and absorption of LPS.8
Loss of Gut Permeability Control
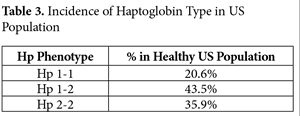 Even when the gut flora is unhealthy, releasing LPS and toxic metabolites, the properly functioning gastric mucosa is normally effective at discrimination and protection. Unfortunately, many factors have resulted in loss of control over gut permeability. Readers will recall my August, 2013 IMCJ editorial, “Zonulin! The Wheat Conundrum Solved (Well, Mostly …).”9 Basically, according to haptoglobin (Hp) type, eating foods with gluten grains (wheat, rye, and barley) results in the release of zonulin, which opens up the tight junctions allowing free entry of gut constituents. As can be seen from Table 3, 79.4% of the US population is homo- or heterozygous for Hp 2, the precursor of zonulin.
Even when the gut flora is unhealthy, releasing LPS and toxic metabolites, the properly functioning gastric mucosa is normally effective at discrimination and protection. Unfortunately, many factors have resulted in loss of control over gut permeability. Readers will recall my August, 2013 IMCJ editorial, “Zonulin! The Wheat Conundrum Solved (Well, Mostly …).”9 Basically, according to haptoglobin (Hp) type, eating foods with gluten grains (wheat, rye, and barley) results in the release of zonulin, which opens up the tight junctions allowing free entry of gut constituents. As can be seen from Table 3, 79.4% of the US population is homo- or heterozygous for Hp 2, the precursor of zonulin.
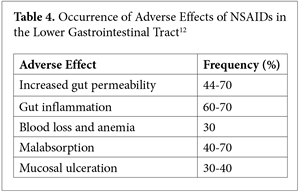 Many other factors cause loss of gut permeability control, including both type 1 and 2 diabetes, excessive alcohol consumption, nonsteroidal anti-inflammatory drugs (NSAIDs)—the list is long.10,11 The later is particularly important, as can be seen in Table 4.
Many other factors cause loss of gut permeability control, including both type 1 and 2 diabetes, excessive alcohol consumption, nonsteroidal anti-inflammatory drugs (NSAIDs)—the list is long.10,11 The later is particularly important, as can be seen in Table 4.
There is also emerging research showing that those with some genetic variations have increased risk of excessive gut permeability. For example, patients with inflammatory bowel disease with mutation in NOD2i are found to have a 75% increased risk of excessive gut permeability. In apparently healthy first-degree relatives, the risk of leaky gut is a significant 40%.13
Not only does excessive gut permeability increase absorption of endotoxins, so does a high-fat diet. This may be part of the reason for upregulation of inflammation after even a single high-fat meal.14
Bottom line, loss of gut permeability control is surprisingly common in our modern age, emphasizing the critical importance of optimal gut flora for health as well as optimizing digestion and healing the gut mucosa.
Conclusion
After spending approximately 100 hours looking at the research, I am convinced the idea of toxins from the gut has substantial clinical relevance. In fact, the more I look at the research, the more I find, suggesting we are only seeing the tip of the iceberg. Once again, the old timers were right.
When speaking to new naturopathic students, I typically start with the admonition, “Read the old timers!” They had remarkable clinical insights. But I warn them to realize that their explanation of what they were seeing was probably wrong, as their understanding was impaired by the very limited physiological research of the time. My other caution is to realize that once a clinician developed an insight that helped a lot of patients, he/she would then go on to inappropriately assert that his/her theory was the cure for all disease. Sadly, as we all know, there is no such thing as one cure for all disease. I have now had the opportunity to delve deeply into the current science that evaluates many of these old concepts. I continue to be amazed by their remarkable clinical acumen. While often the explanation was not validated, their observations and interventions were right on.
One more thought, please: For the past 14 years I have written more than 70 editorials, many on the foundational concepts of natural/integrative/functional medicine. The more research I study (and the more patients I treat), the more clearly I am seeing that all of the fundamentals of health are now so undermined that only treating disease is no longer a viable strategy. In fact, I would assert that the foundations of health must first be reestablished before we can know if a patient’s apparent disease is real or simply the body’s best adaptation to their distorted environment.
In This Issue
As John Weeks so eloquently states, the partnership between the Institute for Functional Medicine and the Cleveland Clinic could indeed be a tipping point. This is a remarkable opportunity to demonstrate in a bastion of conventional medicine that fundamentally changing the way we think about patients is the only real solution to the health care crisis. The leadership of Senator Tom Harkin (D- IA) was pivotal for advancing integrative medicine at the federal level. His retirement, while much deserved, is cause for concern. At the events organized by the integrative medicine community to honor Senator Harkin, I interviewed the many integrative medicine leaders in attendance and recorded the evening ceremony. These will be the basis of my next editorial.
Providing further substance for my editorial, Matthew J. Bull, BSc, PhD, and Nigel T. Plummer, PhD, provide us a 2-part series (Part 2 forthcoming) on the human gut microbiome. The first sentence of their abstract says it all: “The bacterial cells harbored within the human gastrointestinal tract (GIT) outnumber the host’s cells by a factor of 10 and the genes encoded by the bacteria resident within the GIT outnumber their host’s genes by more than 100 times.”
One of the problems with the US Department of Agriculture’s GRAS List (Generally Recognized as Safe) is that food manufacturers appear to have used this as justification/cover for indiscriminately adding large amounts of various chemicals to the food we eat. This has in some cases resulted in unexpected and significant physiological dysfunction. Associate Editor Lara Pizzorno, MDiv, MA, LMT, provides us an in-depth look at how our modern, processed food diet has resulted in excessive intake of phosphorous causing distorted metabolism and increased risk for several diseases.
Managing Editor Craig Gustafson provides us an intriguing interview of one of my heroes, Bruce Ames, PhD, and his colleague Rhonda Patrick, PhD. They discuss Ames’s triage concept, an extremely important understanding of how the body prioritizes nutrient utilization. The first time I heard him lecture on this concept, I was struck by how well it explained disparate research findings.
I suggest you empty your bladder before reading BackTalk by associate editor Bill Benda, MD.
Joseph Pizzorno, ND, Editor in Chief
drpizzorno@innovisionhm.com
http://twitter.com/drpizzorno
References
1. Wikoff, WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698-3703.
2. The Free Dictionary by Farlex. Endotoxin. http://medical-dictionary. thefreedictionary.com/endotoxin. Accessed October 31, 2014.
3. Neves AL, Coelho J, Couto L, et al. Metabolic endotoxemia: A molecular link between obesity and cardiovascular risk. J Mol Endocrinol. 2013;51(2):R51-R64
4. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761-1772.
5. Lassenius MI, Pietiläinen KH, Kaartinen K, et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34(8):1809-1815.
6. Alisi A, Manco M, Devito R, et al. Endotoxin and plasminogen activator inhibitor-1 serum levels associated with nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2010;50(6):645-649.
7. Shah R, Hinkle CC, Haris L, et al. Adipose genes down-regulated during experimental endotoxemia are also suppressed in obesity. J Clin Endocrinol Metab. 2012;97(11):E2152-E2159.
8. Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23): 2989-2995.
9. Pizzorno J. Zonulin! The wheat conundrum solved (well, mostly …). Integrat Med Clin J. 2013;12(4):8-14.
10. de Kort S, Keszthelyi D, Masclee AA. Leaky gut and diabetes mellitus: What is the link? Obes Rev. 2011;12(6):449-458.
11. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42):E4485-E4493.
12. Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15(suppl 3):S3.
13. Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: Role of CARD15 3020insC mutation? Gut. 2006;55(3):342-347.
14. Laugerette F, Vors C, Peretti N, et al. Complex links between dietary lipids, endogenous endotoxins and metabolic inflammation. Biochimie. 2011;93(1):39-45.
All rights reserved. Terms and Conditions.

